Chemical engineering's Knowledge
Menu bar
Friday, June 19, 2020
Petrochemical feedstock sources
The adjacent diagram schematically depicts the major hydrocarbon sources used in producing petrochemicals are:
Methane, ethane, propane and butanes: Obtained primarily from natural gas processing plants.
Naphtha obtained from petroleum refineries.
Benzene, toluene and xylenes, as a whole referred to as BTX and primarily obtained from petroleum refineries by extraction from the reformate produced in catalytic reformers.
Gas obtained from petroleum refineries.
Methane and BTX are used directly as feedstocks for producing petrochemicals. However, the ethane, propane, butanes, naphtha and gas oil serve as optional feedstocks for steam-assisted thermal cracking plants referred to as steam crackers that produce these intermediate petrochemical feedstocks:
- Ethylene
- Propylene
- Butenes and butadiene
- Benzene
In 2007, the amounts of ethylene and propylene produced in steam crackers were about 115 Mt (megatonnes) and 70 Mt, respectively.The output ethylene capacity of large steam crackers ranged up to as much as 1.0 – 1.5 Mt per year.
Steam crackers are not to be confused with steam reforming plants used to produce hydrogen and ammonia.
Important note for Extraction process
The main advantage of using mixing or some type of mechanical energy, compared to packed plate or spray columns, is the ability to get a smaller volume for the same degree of extraction. However, if an attempt is made to use too much energy, then problems of settling characteristics are encountered, and this negates the advantages of the mixed system many times.
In the mining industry, it is quite typical to use mixer settlers. These usually involve an extraction step, a scrubbing step, and then a stripping step. Usually the requirement is for only one or two stages in each of these are with the use of very selective ion exchange chemicals in the system. To eliminate interstage pumps a pump-mixer is used in which some of the head component of the impeller is converted to a static head so that fluids can be pumped against small static heads in the mixers and settlers of the whole train. This has worked well in many applications, although there is a potential problem that the conditions required for effective pumping are not optimum for the mixing that is required in the mixing stage, and there may be some design parameters that are difficult to satisfy in the systems.
The other area is the counter current liquid- liquid extraction column.Using mixer stages separated by stationary horizontal discs. These have the advantage of only one interface for settling to occur, plus the fact that solids can be handled in one or both phases. Also, all the principals of fluid mixing can be used to design an effective transfer system.
One of the key variables to be studied in the pilot plant is the effect of turn down ratio, which is the ratio of flow to the design flow through the column, so that predictions can be made of performance during reduced throughput during certain parts of the plant processing startup.
Sunday, May 31, 2020
Plate Columns and Comparison of Tray Types
For purpose of distillation, plate columns and packed columns can be used. In plate columns each plate constitutes a single stage, or in packed columns where mass transfer is between a vapor and liquid in continuous counter current flow.
In order to design a plate type distillation column, following factors must be considered:
1) The type of plate or tray
2) The vapor velocity, which is the major factor that determines the diameter of the column.
3) The plate spacing, which is the major factor fixing the height of the column when the number of stages is known.
Types of Trays:
The main requirement of a tray is that it should
a) provide an intimate contact of vapor and liquid phases, because more the contact of these two, more will be the mass transfer which brings about more enrichment.
b) it should be capable of handling more desired flow rates of vapor and liquid without excessive entrainment or flooding.
c) be stable in operation or have flexibility in operation.
d) be reasonably easy to erect and maintain.
Arrangement of Flow:
The arrangements for the liquid flow over the tray depend largely on the ratio of liquid to vapor flow. Three layouts are shown here, of which the cross-flow array is much the most frequently used.
a) Cross-Flow: Normal, with a good length of liquid path giving a good opportunity for mass transfer.
b) Reverse: Downcomers are much reduced in area, and there is a very long liquid path. This design is suitable for low liquid -vapor ratios.
c) Double-pass: As the liquid flow splits into two directions, this system will handle high liquid-vapor ratios.
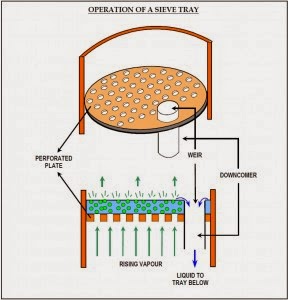
The liquid reflux flows across each tray and enters the downcomer by way of weir, the hight of which largely determines the amount of liquid on the tray. The downcomer extends beneath the liquid surface on the tray below, thus forming a vapor seal. The vapor flows upwards through risers into caps, or through simple perforations in the tray. Weir and downcomer is shown in second figure as follows:
Types Of Trays:
Purpose of tray is to provide an intimate contact of liquid and vapor, and to make a low drop of pressure. So far in industry, following three types of trays are usually used:
a) Seive or Perforated Trays:
These are much simpler in construction, with small holes in the tray. The liquid flows across the tray and down the segmental downcomer. This type of tray offers a very low pressure drop and is cheaper than the rest of the two, but it brings about less vapor-liquid contact as compared to the other two.
The general form of the flow on a sieve tray is typical of a cross-flow system. With the sieve plate the vapor velocity through the perforations must be greater than a certain minimum value in order to prevent the weeping of liquid stream down through the holes. At the other extreme, a very high vapor velocity leads to excessive entrainment and loss of tray efficiency.
b) Bubble Cap Trays:
This is the most widely used tray because of it's range of operations, but is now-a-days unable to compete with the third type which offers more flexible operation. The individual caps are mounted on risers and have rectangular or triangular slots cut around their sides. The caps are held in position by some form of spider, and the area of the riser and the annular space around the riser should be about equal. With small trays, the reflux passes to the tray below over two or three circular weirs, and with the larger trays through segmental downcomers.
This type of tray provides good efficiency than seive tray, flexible in operation (i.e, can be used for a range of liquid-vapor flow rates) but is most costly and offers great pressure drop as compared to the other two. Bubble cap trays are capable of dealing with very low liquid rates and are therefore useful for operation at low reflux ratios.
c) Valve Trays:
These may be regarded as a cross between a bubble-cap and a sieve tray. The construction is similar to that of cap types, although there are no risers and no slots. It may be noted that with most types of vlave tray the opening may be varied by the vapor flow, so that the trays can operate over a wide range of flowrates (i.e, it provides more flexibility in operation than a bubble-cap tray).
It is low is cost than a bubble- cap tray since it is simple in construction. Because of their flexibility and low price, valve trays are tending to replace bubble-cap trays. It operates at the same capacity and efficiency as sieve trays. It has high turn-down ratio, i.e, it can be operated at a small fraction of design capacity.
Saturday, May 30, 2020
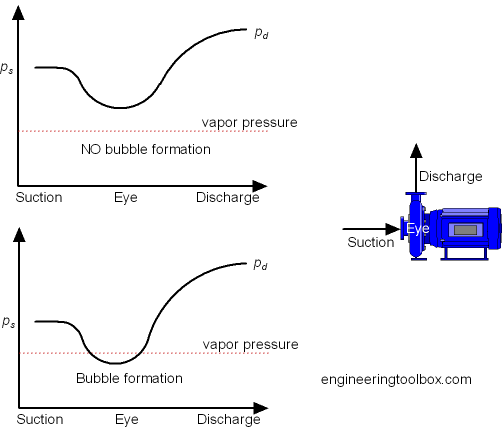
Pump Net Positive Suction Head and Its Types
NPSH is actually pressure at the suction of a pump. It must be positive for the pumping process to continue. It determines whether the pump will loose suction or not. It is the sum of
1) static pressure (act in positive)
2) pressure head (acts in positive)
3) suction lift (acts in negative)
4) vapor pressure of pumping fluid at pumping temperature (acts in negative hence it comes with negative sign)
NPSH = static pressure + pressure head - suction lift - vapor pressure
If this value results in a negative number (i.e, suction lift and vapor pressure are more than the sum of static pressure and pressure head), then the pump will loose suction and pump will no longer pump the liquid. You need to increase static pressure and/or pressure head, and/or you can decrease suction lift and/or vapor pressure.
If this value results in a positive number, then the pump will continue to pump the liquid.
An air gap comes when pump looses the suction and since the pump does not make the air flow, the liquid will remain static. Running the pump in this condition for a long time would result in damage to the pump.
Types of NPSH:
NPSH is of two types:
1. NPSH available (NPSHA):
It is the pressure "actually present at the suction" of an installed pump calculated on the site using the same above equation.
2. NPSH required (NPSHR):
It is the "minimum pressure that must be present" at the suction of the pump to pump the liquid at desired flow rate. If the suction pressure is less than NPSHr, the pump will no longer pump the liquid.
Thursday, May 28, 2020
Functions of Tray In Distillation Column
One of the most prominent hardware used for mass transfer is tray. Tray columns are widely used in various types of mass transfer operations. All the simulation results, which predict a certain number of theoretical stages, can be converted to actual trays depending upon tray efficiency for a particular service.
Basic functioning of a tray/plate is mass transfer. It actually brings about vapor-liquid contact. More the vapor-liquid contact, more would be the mass transfer. This is practically achieved when a liquid is held on a perforated tray and vapors pass through this liquid layer from below the liquid depth through the perforations. It should be noted here that, flow rate should be adjusted such as the liquid "may not" come down from the tray above "through perforations". If this happens, then vapor-liquid contact would be less which will result in low vapor liquid contact and hence it lowers the efficiency of tray. This condition is said to be weeping.
Similarly, vapor velocity must not be so high, since it may take the liquid over the tray to the tray above it, in form of droplets. This will again reduces the efficiency of the tray in the similar manner. This process is called entrainment.
In any conventional tray vapour rises through the liquid pool on the tray deck and then disengages from the liquid in the space above the deck. Liquid enters the tray from a downcomer above and leaves via a downcomer below.
Conventional Tray has three functional zones:
- Active area for mixing vapour and liquid: This is the zone where mass transfer occurs.
- Vapour space above the active area: This is the zone in which liquid is separated from vapour.
- Downcomer between trays. This zone has two functions, first moving liquid from one contacting tray to another and second disengaging vapour from liquid.
Natural gas field processing/ Gas dehydration
1. Hydrates and Hydrate Composition
A hydrate is a physical combination of liquid water
and other smaller molecules to produce a solid which has an ice-like or dirty
wet-snow appearance but possesses a different structure than ice.
They are 90% (by weight) water; the other 10% is
composed of one or more of the following compounds: methane, ethane, propane,
iso-butane, n-butane, nitrogen, carbon dioxide and hydrogen sulphide.
The simplified structure of the hydrate crystal is
water molecules with the smaller hydrocarbon molecules occupying spaces between
the water. In a sense, the water molecules trap the hydrocarbons in a crystal
lattice.
Hydrates have specific gravities ranging from 0.96 to
0.98 and therefore float on water and sink in liquid hydrocarbons.
Hydrates are found in most areas of a gas producing
operation:
-
Downhole,
-
Gathering systems
-
Processing systems
Hydrates are a constant challenge in producing natural
gas and must be considered in all designs and production practices.
2. Hydrate Formation
Dangers
and problems created by Hydrates
An obstruction caused by a hydrate formation
can:
a)
reduce or block flow
b)
increase back pressure
c)
increase differential
pressure through a process
The pressure differential across a hydrate “plug” can cause it to
break loose and travel down the pipe at
very high velocities. People have been killed by hydrate plugs dislocating and
smashing through process equipment.
Hydrates can form single or multiple plugs in
lines.
The
formation of hydrate plugs can cause pressure to be trapped between two plugs.
Once formed, hydrates cause a threat to
personnel and equipment if not handled properly during removal. Pressure on both sides of a plug must be
reduced to keep it from dislocating and moving to the low pressure side.
The conditions that affect hydrate
formation are:
®
Pressure
®
Temperature
®
Composition
®
Gas must be at or below its
water dew point or saturation
condition
Note that when the natural gas is in the reservoir, it is assumed to be in
contact with water at equilibrium. The
gas is saturated with water. Therefore, even if free water is not present,
changing the conditions (pressure or temperature) can cause free water to form.
An example of changing the conditions would be passing the gas through a choke.
Pressure
and Temperature
Hydrates tend to form when the pressure
is high and the temperature is low.
All compositions have different points at which hydrates will form. The
most important point to remember is that it does not have to be colder than 0oC in order to have the
conditions necessary for hydrate formation.
When the formation of a hydrate causes a
restriction in a flow line, the restriction causes a pressure drop. The
resulting expansion of gas causes a cooling of the gas (Joule-Thomson effect).
This scenario is referred to as auto-refrigeration and can cause the
further growth of hydrates until the flow is blocked completely.
Hydrates often form at chokes, orifices, thermowells, bends in pipe etc.
3. Composition
The composition of the gas has a large effect on the formation of hydrates.
e.g. A small amount of propane or iso-butane in methane will cause hydrates
to form at warmer temperatures than in pure methane alone.
The presence of H2S will also affect the formation of hydrates.
When H2S is present in a gas composition, it will result in the
formation of hydrates at warmer temperatures at a given pressure.
The presence of CO2 has a much smaller impact and often reduces
the hydrate formation temperature at fixed pressure for a hydrocarbon gas
mixture.
4. Hydrate Inhibition
It is convenient to divide hydrate formation into two categories:
1)
Hydrate formation due to a
decrease in temperature with no sudden pressure drop, such as in the flow
string or surface line, and
2)
Hydrate formation where a sudden
expansion occurs, such as in flow provers, orifices, back-pressure regulators
or chokes.
A review of the conditions that tend to promote
the formation of natural gas hydrates are:
1)
Natural gas at or below
its water dew point with liquid water present.
2)
Temperatures below the
“hydrate formation” temperature for the pressure and gas composition
considered.
3)
High operating pressures
that increase the “hydrate formation” temperature.
4)
High velocity or agitation
through piping or equipment.
5)
Presence of a small “seed”
crystal of hydrate.
6)
Presence of H2S
or CO2 is conducive to hydrate formation since these acid gases are
more soluble in water than hydrocarbons.
Now, if you consider the first 2 points and the
second 6, some of the techniques which could be used to inhibit the formation
of hydrates are:
·
Raise the temperature so
it doesn’t hit its hydrate formation temperature
·
Lower the pressure so it
doesn’t hit its hydrate formation temperature.
·
Remove the water so it
doesn’t hit its water dew point conditions.
These techniques are all used to some degree.
Line heaters are used to keep the gas temperature high. Keeping the pressure
low is often not an option but restrictions which cause a sudden pressure drop
and subsequent drop in temperature are avoided. Dehydration units are used at
the wellsite and at plants to remove the water. Another technique used to lower
the hydrate formation temperature is:
·
Chemical injection to
depress hydrate formation temperature.
Many chemicals depress the temperature at which
hydrates and/or ice form. Ammonia and brine were used in the past, but the
current choice is either a glycol or methanol. Methanol is the common field
choice for our situations and glycol would tend to be used in a plant refrigeration
system.
4. Dehydration - Overview
Ø removal of water associated with the production of natural gas.
Ø prevents hydrates and reduces corrosion.
Ø Prepares gas for further processing (e.g. cryogenic)
Ø Removal of free water to prevent accumulations and promote single-phase
pipeline flow.
·
Three major methods of gas
dehydration are commonly used:
a) Absorption (wet)
ii) diethylene
glycol (DEG)
b) Adsorption (dry)
i) molecular
sieves (commonly called zeolites)
ii) silica
gel (essentially SiO2 in bead or powder form)
iii) activated
alumina (in extrudate or pellet form)
c)
Low Temp Processes
i)
Processes that
intentionally form and melt hydrates (LTX)
ii)
Processes that use hydrate
inhibitors
iii)
Processes that use
mechanical refrigeration
4.1. Absorption
·
dehydration by glycol
absorption is one of the most common methods of dehydration used to bring the
water content of a gas stream to pipeline spec.
·
a common dehy unit
consists of a absorption tower (contactor) in which wet gas is contacted with
lean glycol and a stripper in which heat is used to remove water from the rich
glycol.
·
glycols are used for
dehydration as water and glycol are mutually soluble in the liquid phase. Water
boils at a lower temp than glycol, so “rich” glycol is heated to a temperature
above the boiling point of water but below the boiling point of the glycol.
This creates “lean” glycol.
·
Commonly used in the field
at a wellsite for hydrate and free water protection, or in a plant handling
greater volumes of gas for final pipeline spec drying.
·
The advantages of a glycol
unit over a solid desiccant unit are:
a.
Lower installed costs
b.
Lower pressure drop in the
contacting tower
c.
Continuous process rather
than batch
d.
Glycol make-up is simple
vs. recharging dry beds
e.
Glycol units require less
make-up heat to regenerate
f.
Glycol systems operate in
the presence of contaminants that would foul a solid desiccant.
g.
Glycol systems are
adequate at water removal for most spec’s (except cryogenic processing).
·
The disadvantages of a
glycol unit are:
a.
Very low water dew-points
cannot be achieved
b.
Glycol is susceptible to
contamination
c.
Glycol is corrosive when
contaminated or decomposed.
4.2. Process Description
Gas Stream:
a.
Gas enters inlet separator
and liquids are removed.
b.
Gas enters contacting tower
and starts upward through a chimney tray.
c.
The gas passes through the
trays or packing, contacting glycol as it travels. The water in the gas has an
affinity for the glycol and attaches to it.
d.
The gas exits the tower
through a mist eliminator to the next process or to the flowline.
Glycol Stream:
a.
Lean glycol in the
accumulator is pumped to the top of the contactor tower.
b.
The glycol picks up water
from the gas as it travels down from tray to tray (or through the packing).
c.
The glycol exits as rich
glycol, is warmed through the accumulator and is dumped into the stripping
column of the reboiler.
d.
The rich glycol is heated
in the reboiler by a natural gas flame in a burner tube. The temperature causes
the water to vaporize and exit the stripping tower (where it contacts more
incoming rich glycol).
e.
The lean glycol spills
over into the accumulator ready for its next pass through the contacting tower.
4.3. Dehydrator Components
Inlet Scrubber
- simple two phase separator used to remove
water and liquid hydrocarbons from the wet gas stream.
Glycol - Gas
Contactor
-
vessel in which wet gas is
contacted with lean glycol.
-
utilizes counter-current
flow: gas upward and lean glycol downward.
-
trays exist in the
contactor (valve or bubble cap) to increase the contact time between the wet
gas and lean glycol.
-
mist extractor is located
at the top of the vessel to remove any glycol entrained in the gas.
-
lean glycol is pre-cooled
by a double pipe heat exchanger prior to entering the vessel (glycol can absorb
more water at cooler temps).
-
Glycol should enter the
dehy at about 10o hotter than the gas temperature. If it is too hot,
it can lead to foaming and inefficient dehydration, and if it is too cold,
hydrocarbons can condense in the glycol.
Filter and Pump
-
rich glycol exits the bottom of the contactor and is
filtered prior to entering a pump.
-
filter removes solid
components from glycol in order to protect the pump and decrease operational
problems with fouling of the dehy. unit.
-
the hydraulic pump
utilizes rich glycol from the contactor as the power fluid to pump lean glycol
from the surge tank to the contactor.
Stripping Still
-
the warm rich glycol enters the stripping still
after being preheated in a heating coil (tube side) in the surge tank.
-
The stripping still is usually filled with ceramic
packing or structured packing to improve the surface area contact of the water
vapour with the rich glycol.
-
allows any glycol vapors
to be condensed to eliminate losses.
-
water vapor exits the top
of the stripping still.
-
Fins on the stripping
still condenser section cool the vapor to help drop out any glycol which may be
entrained or vaporized in the water vapor. A temperature just above the boiling
point of water is optimal for the condensing section.
Reboiler
-
vessel in which rich glycol is heated to 175 – 200oC
to vaporize water.
-
heat source is a natural
gas flame in a fire tube.
-
glycol is regenerated to
99% glycol.
-
Glycol regeneration
efficiency can be improved with a “Stahl column”
-
Dry stripping gas (usually
fuel gas) is injected into a column between the reboiler and accumulator
-
The dry gas mixes with the
lean glycol (99%) and strips out more water. Glycol concentrations of 99.9% can
be achieved.
-
The gas enters the
reboiler and exits with the water vapour.
-
The cost to operate a
Stahl column must be weighed against the benefits. The costs are fuel gas (and
hydrocarbon to the environment).
Heat
Exchange/Surge Tank
-
regenerated glycol leaves the reboiler through an
overflow pipe and enters the shell side of the Surge Tank.
-
the lean glycol is cooled
by the rich glycol on the tube side of the exchanger.
-
the surge tank is a liquid
accumulator for the glycol pump to ensure the pump receives a uninterrupted
supply of glycol.
-
A sweet gas blanket is
often maintained in the vapour space above the glycol in the accumulator. A
slight pressure is held which prevents oxygen from the atmosphere and water
vapour from the reboiler from entering.
*
TEG is by far the most common glycol used in dehy’s (probably 90 to 95%). DEG
may be used in fields where minimal gas is available for heating the glycol;
DEG does not have to be heated to as high a temperature to release the water.
Subscribe to:
Comments (Atom)